
Some late complications showed up after Food and Drug Administration approval, so it was taken off the market.)ĭr. And, in fact, we’ve removed some of them just to prevent future problems with the implants.” (The CyPass glaucoma implant from Alcon suffered the same fate. In the end, the FDA issued a warning saying that no more of these should be implanted.
#Raindrop eye surgery reviews trial#
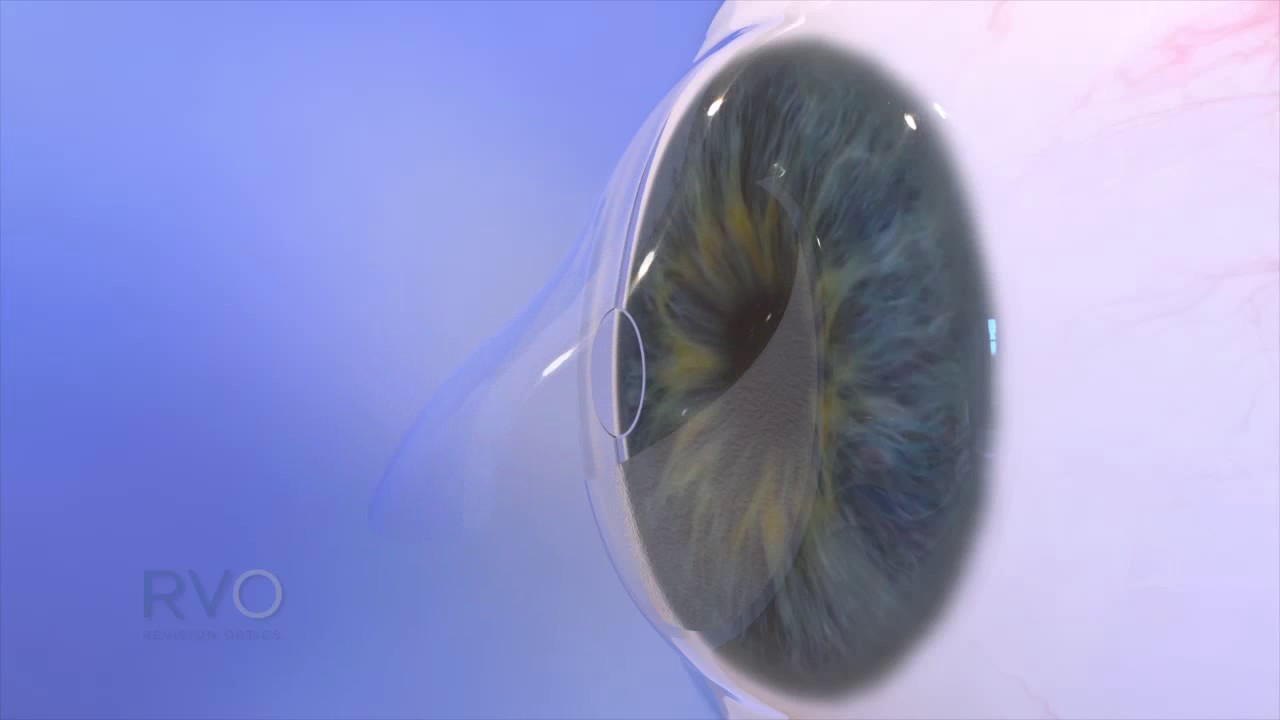
In my practice, I did about 30 of the Raindrop implants, and the vast majority of my patients did very well, but not everyone in the trial did. The hydrogel material was probably not as biocompatible as another material might have been, though many patients did well. “We think the problem was the material,” he says. Hovanesian adds that, with ReVision Optics, both the company and the technology ultimately failed, despite a respected team and a promising product. And so, while it actually did achieve FDA approval, it was pulled off the market.”ĭr. “Additionally, it had decentration issues. “That particular product was removed from the market because it had some adverse outcomes, primarily related to interface haze between the hydrogel and the cornea,” Dr. It’s a hydrogel that was placed under a flap to cause a central steepening of the cornea and basically create a multifocal cornea. Richard Lindstrom, MD, who is in practice in Minneapolis, notes that another corneal inlay has been taken off the market: the Raindrop from ReVision Optics. I don’t think the company has any grand designs that it’s going to take over the world of presbyopia correction, but I believe it’s a valuable offering they will continue to make available.” Kamra to be a blockbuster product for them. “But CorneaGen has a wide variety of products that appeal to cornea specialists, and they don’t need “If the whole company’s existence depended upon just Kamra, it would be hard to keep a company going on a small product like that,” he says. Hovanesian believes that Kamra will stick around because the company is set up to support it. Though the inlay’s adoption rate by surgeons isn’t anything like when PRK or LASIK were first approved, Dr.

Kamra corneal inlay is the only one still available. Several corneal inlays have come to market in recent years, but the So, we have to overcome both fear and cost concerns with corneal inlays.” In some cases, the patient may have hyperopia or astigmatism, so he or she may have to undergo a refractive procedure as well. In addition, the cost of a Kamra inlay, although it’s in only one eye, is greater than the cost of LASIK. Most people who have not tried monovision have a negative reaction to the idea when you present it. That causes patient uncertainty about how well it will work for them. Additionally, it’s a device that requires patients to adapt to some degree of monovision, because it’s only in one eye. In the case of fear, I don’t think patients are much more scared than they would be of LASIK, but most people don’t know anyone who has a corneal inlay. “As we know from experience, the two biggest barriers to LASIK are fear and cost,” Dr. He adds that there are several barriers to patient adoption of corneal inlays. “There were good companies and, in the case of Kamra, good products, but there has not been widespread acceptance,” he says. According to John Hovanesian, MD, who practices in Laguna Hills, California, it’s clear that widespread market adoption of corneal inlays has not yet happened. T he future for synthetic corneal inlays appears bleak.


 0 kommentar(er)
0 kommentar(er)
